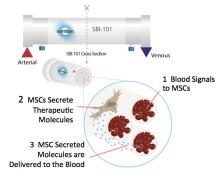
Our latest featured trial is NCT03015623, which was registered in January 2017 but started recruiting patients in June 2017. In this trial, patients with acute kidney injury have their blood filtered through a device that holds mesenchymal stem/stromal cells from allogeneic bone marrow.