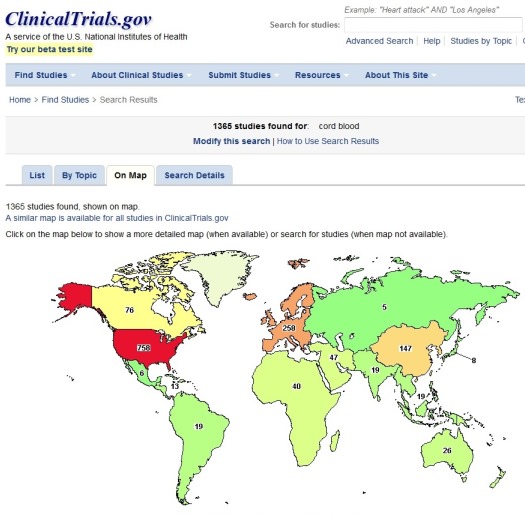
 We have all had this experience: You go to a conference, and a speaker gets up and says, “I checked ClinicalTrials.gov this morning, and there are 1365 trials with cord blood!” (as of 28 February 2017). How many clinical trials are really performing cell therapies with cord blood? We will drill down through the process to find that answer, and explore how many alternative answers may exist.
We have all had this experience: You go to a conference, and a speaker gets up and says, “I checked ClinicalTrials.gov this morning, and there are 1365 trials with cord blood!” (as of 28 February 2017). How many clinical trials are really performing cell therapies with cord blood? We will drill down through the process to find that answer, and explore how many alternative answers may exist.
What the speaker probably did is pull up the web page ClinicalTrials.gov, typed the keywords “cord blood” into the search box, and received a message that 1365 entries contain these keywords. Some of these entries are not even studies about cord blood, they simply happen to contain the keywords somewhere in the entry.
The main feature of keyword searches in ClinicalTrials.gov, or any of more than a dozen other clinical trial registries that you can find through the WHO on-line portal, is that these searches are cumulative. You are getting hits for every trial containing the keywords “cord blood” that was ever registered since ClinicalTrials.gov was launched in the year 2000. Obviously, many of these trials have long since been terminated or completed.
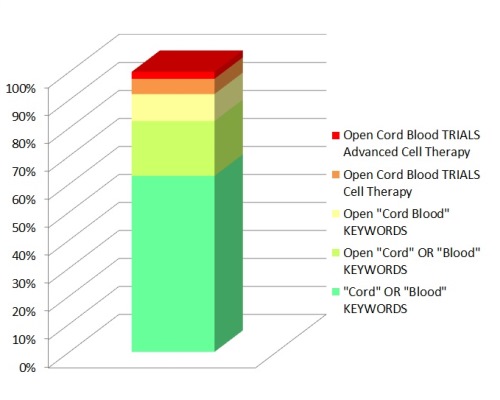
The Advanced Search option on Clinicaltrials.gov allows the user to select only those trials that contain the keywords “cord blood” and that have recruiting status “Open”. This reduces the number of hits from 1365 to 425. Many users of ClinicalTrials.gov do not realize that if you simply type “cord blood” into the search box, you get all trials that contain either “cord” or “blood”. In order to only select for trials with the exact phrase “cord blood” you must put the phrase into quotes before searching. This further reduces the number of Open trials to 209. Nonetheless, keyword searches do not discriminate between different types of clinical trials. The only way to really know what each trial is doing is for a human scientist to read every one.
Our team checked each of the 209 entries in ClinicalTrials.gov that are supposedly open cord blood trials, and found that only 118 of these trials are conducting cell therapy with cells from cord blood, either alone or in combination with other cell therapies. Under the category of “open trials” we included trials that are recruiting, not yet recruiting, pending, ongoing, and enrolling by invitation, but excluded follow-up studies. This analysis took weeks of effort, but in the end revealed that for the exact phrase “cord blood”, only 56% of the keyword hits are active cell therapy trials.
There are two main formats in which analyses of clinical trials have been produced. One business model is that academics analyze the trials and then publish papers that present summaries of the data. In this model the results are free because the academics had some form of institutional support to do the work. The second main business model is that biotech consultants compile reports and sell them to clients, getting financial support at the end of the process. Some biotech companies give away certain data sets as a teaser to advertise their services.
We have launched a third format of providing cell trials data at CellTrials.org. Like biotech consultants, we have compiled the data in advance and are selling the files. Like academics, we publically reveal our search criteria so that our results can be compared to the results in other reports. We even provide a comparison table of all public results. What is unique to our data product is that we provide raw data, so that the end users can sort and select it however they wish.
Why is it that a keyword search of ClinicalTrials.gov yields 209 open trials for the exact phrase “cord blood”, whereas a scientific review only finds 118 open trials performing cell therapy with cord blood? To find the answer, we explored the breakdown of the 209 entries for open cord blood trials. It turns out that 22% of these trials are obstetrics studies that use cord blood to measure maternal-fetal health, 31% are traditional hematology/oncology cell therapy, 26% are advanced cell therapy, and 21% are other studies or miscellaneous false positives.
 Any clinical trial that relies on the action of stem progenitor cells and somatic cells is performing “cell therapy”. However, published reports have shown that in recent years about 40% of cell therapy trials are hematology/oncology studies in which the cell therapy is a conventional hematopoietic stem cell transplant (HSCT). A study of trials over the two decades 1992-2012 found that only 23%1 of cell therapy trials were "regenerative", leaving the reader to infer that the remainder were conventional. Studies of more recent time frames found that over the decade 2000-2010, 37%2 of cell trials were "conventional grafts", and cumulative through the end of 2014, 46%3 of open cell trials were "oncology".
Any clinical trial that relies on the action of stem progenitor cells and somatic cells is performing “cell therapy”. However, published reports have shown that in recent years about 40% of cell therapy trials are hematology/oncology studies in which the cell therapy is a conventional hematopoietic stem cell transplant (HSCT). A study of trials over the two decades 1992-2012 found that only 23%1 of cell therapy trials were "regenerative", leaving the reader to infer that the remainder were conventional. Studies of more recent time frames found that over the decade 2000-2010, 37%2 of cell trials were "conventional grafts", and cumulative through the end of 2014, 46%3 of open cell trials were "oncology".
At CellTrials.org, we are focused on tracking Advanced Cell Therapies. These are therapies in which cells are more than minimally manipulated, and/or their action is not homologous. Advanced Cell Therapy encompasses most forms of regenerative medicine, it includes stem cell transplants with cells that are more than minimally manipulated, and it includes the new fields of immunotherapy with cells and gene therapy with cells, even tissue engineering grafts that are seeded with cells.
Our team looked at the distinction between general cell therapy and advanced cell therapy for the cord blood trials registered on ClinicalTrials.gov in 2016. During that year, 39 trials were registered using cells derived from cord blood for cell therapy, but 44% of those trials are hematology/oncology studies in which the cord blood is used for conventional HSCT, while 56% are advanced cell therapy.
We now have multiple alternative answers for how many clinical trials there are with cord blood, within the registry Clinicaltrials.gov. Based on the keywords “cord blood”, there are 1365 trial entries, of which 425 are open trials about “cord” or “blood”, but there are only 209 open trials with the exact phrase “cord blood”. Based on our scientific review of each trial, there are 118 open trials performing cell therapy with cord blood, and among them only 54 trials are performing advanced cell therapy with cord blood.
When industry reports are written about the cell therapy sector, many of them try to sound more impressive by claiming the largest possible number of current trials. Rumor has it that the leaders of biotech companies prefer to purchase industry reports that give inflated numbers of trials, because it makes their potential market sound bigger when seeking venture capital. Does anyone who works or invests in the cell therapy industry care about accurate numbers of trials that are novel therapies? If yes, visit us at CellTrials.org.
References
- Li MD, Atkins H, Bubela T. Regenerative Medicine 2014; 9(1):27-39
- Culme-Seymour EJ et al. Regenerative Medicine 2012; 7(4):455-462
- Heathman TRJ et al. Regenerative Medicine 2015; 10(1):49-64