Alexey Bersenev, MD PhD, & Frances Verter, PhD
This map shows the number of companies in each country that are developing allogeneic CAR-immunotherapies. The top of the scale is 38 in the United States.
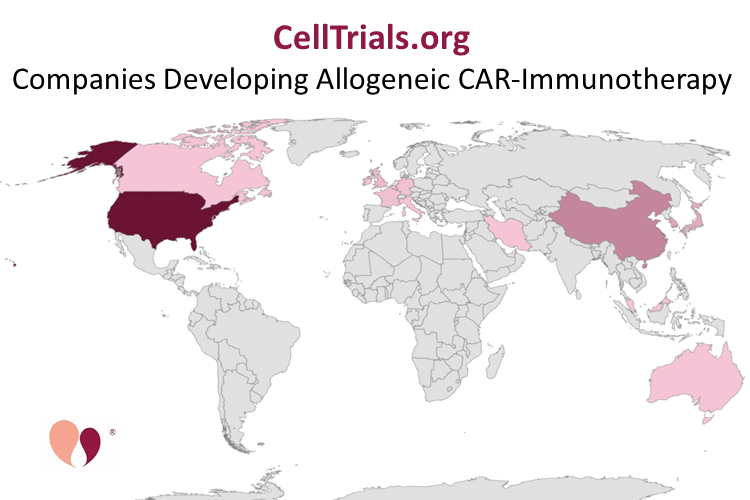
At Celltrials.org, we maintain international databases of companies that are developing any form of cellular immunotherapy or specifically CAR-immunotherapy. At the beginning of 2020, we started including information about whether companies were using autologous cells or developing allogeneic cellular immunotherapy. We also display an interactive map of cellular immunotherapy companies. However, because the information in the interactive map is displayed as percentages in each country, it does not show at a glance how many companies there are, and where they are located. The map above is not interactive, but it displays the actual numbers of companies in each country that have allogeneic CAR products.
Our Aug 2020 database holds 396 companies worldwide that are developing one or more forms of cellular immunotherapy. Among them are 10 companies that operate in two countries. The majority of companies working in cellular immunotherapy are in the United States (165 companies) or China (127 companies). However, that leaves 114 companies operating in 26 other countries, so the landscape of work in cellular immunotherapy is definitely multi-national.
The heat map above only displays the 80 companies that claim to be developing CAR-immunotherapy from allogeneic donors. This includes any CAR products, both with T-cells and NK cells, and any company that has one allogeneic product. There are 38 companies in the United States, 14 in China, and 28 companies in 13 other countries. There may be more such companies that have not revealed their work. These represent 35% of the 230 companies that we list in various countries that are developing CAR-immunotherapy.
We have emphasized allogeneic CAR-immunotherapy because this topic is very popular with our readers. The first market approvals for cellular immunotherapies were for the autologous CAR-T products Kymriah (from Novartis) and Yescarta (Kite Pharma) in 2017. Since then, competition has been growing to develop allogeneic CAR-T products that are less expensive, faster to produce, and more reliable. However, the full picture of cellular immunotherapy is much more complex than just CAR products. There are allogeneic immunotherapy products in development which are based on the cell types DC, MSC gene modified, NK, TCR, VST, and γδ T-cells.