Posted February 2019:
CellTrials.org is the only database in the world that compiles advanced cell therapy clinical trials from all national registries, not just ClinicalTrials.gov. We are currently compiling trials from the following registries:
| Country | Clinical Trial Registry |
| United States | ClinicalTrials.gov |
| Australia & New Zealand | Australian New Zealand Clinical Trial Registry (ANZCTR) |
| Brazil | Registro Brasileiro de Ensaios Clínicos (RBR) |
| China | Chinese Clinical Trial Registry (ChiCTR) |
| EU | EU Clinical Trials Register (EudraCT) |
| Germany | Deutsches Register Klinischer Studien (DRKS) |
| India | Clinical Trials Registry-India (CTRI) |
| Iran | Iranian Registry of Clinical Trials (IRCT) |
| Japan | Japan pharmaceutical Information Center Clinical Trials Information (Japic) |
| Japan | Japan Medical Association Clinical Trial Registry (JMA-CTR) |
| Japan | Japan University hospital Medical Information Network Clinical Trial Registry (UMIN-CTR) |
| Netherlands | Netherlands Trial Register (NTR) |
| South Korea | Clinical Research Information Service from South Korea (CRiS) |
| Thailand | Thai Clinical Trials Registry (TCTR) |
| UK | International Standard Randomised Controlled Trials (ISRTCN) |
| WHO | World Health Organization (TrialSearch) |
The process of checking over a dozen registries every month is very time consuming. Our team does this manually, with scientists searching by keywords and examining each trial to determine if it meets the criteria for advanced cell therapy. The ratio of trials examined to trials accepted is over ten to one. Many others have developed software to search ClinicalTrials.gov automatically, but at this time no one has software that works on all the trial registries. Moreover, simply collecting trials by keyword search results in a wildly inaccurate compilation. We have previously published blogs1-3 to validate the ratios of real trials versus false positives that are acquired with various keyword searches. We also check for duplications where the same trial is posted to two registries. Compiling a database like ours is not a project that a graduate student can do in a week or two.
When we started compiling monthly data in early 2017, the fraction of our trials that were not included in ClinicalTrials.gov was about 20%. Since we are scientists, we thought it was a big deal that all the other compilations on the market had a 20% error. We keenly remember our first phone conference with a potential customer, the CEO of a company that created industry reports. The CEO dismissively said, “Who cares about 20%?” It was a shock to us, but we have come to learn that companies that only do business in the US simply do not care about the international trials that are missing from ClinicalTrials.gov.
Today, cell therapy has become an increasingly international endeavor. More and more countries are establishing their own registries of clinical trials. For example, Japan has three clinical trial registries, and the oldest pre-dates ClinicalTrials.gov. In any given country, researchers are obligated to list their trials on their own national registry, and they may or may not make the additional effort to cross-post the trial to ClinicalTrials.gov. In fact, we sometimes see trials that we suspect were purposely not cross-posted precisely so that the researchers could keep their work un-noticed.
When we look back at the past two years of international trials in advanced cell therapy, we see that the monthly fraction of clinical trials outside ClinicalTrials.gov has risen 65%, from an average of 23% in 2017 to 38% in 2018.
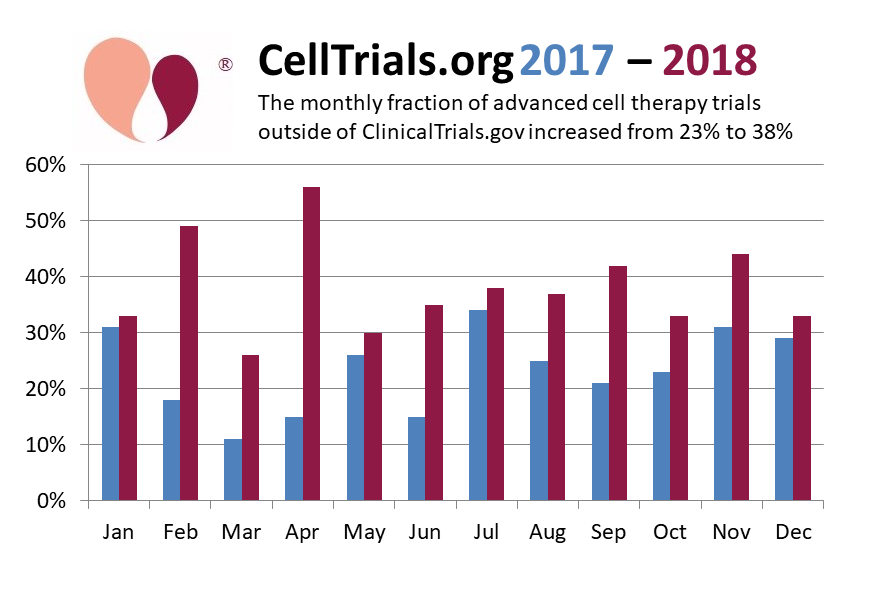
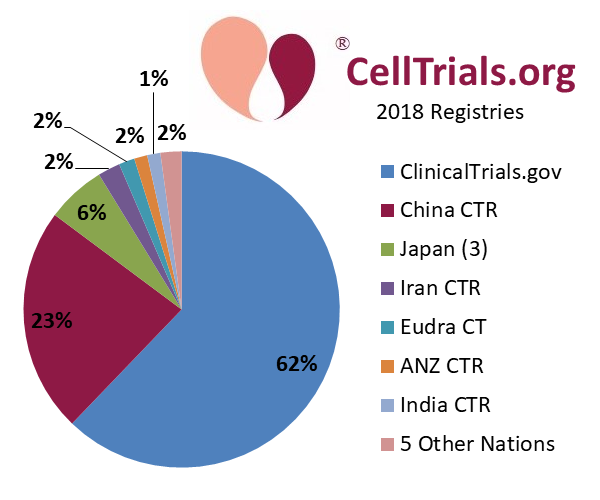
Some people assume that the trials that are not cross-posted to ClinicalTrials.gov are all in China, but this is not correct. For the year 2018, we display a pie chart of the registries on which we found advanced cell therapy clinical trials. During 2018, 62% of trials in advanced cell therapy were posted or cross-posted to ClinicalTrials.gov, another 23% were posted on the ChiCTR registry, and the remaining 15% were posted on 12 other national registries.
There is a growing audience of people in the cell therapy industry who need to know about the trials outside ClinicalTrials.gov. For example, a pharma company that is planning to run clinical trials for a new cell therapy product needs to know if someone else has already run trials to treat their target indication with the same type of cells. From a regulatory standpoint, new cell therapy products are developed and approved in national silos. Nonetheless, if the product is intended to eventually go on the international market, it is helpful to know if a company on the other side of the world already has a similar product that is ahead in the approval pipeline. For example, the first CAR-T therapies Kymriah and Yescarta were quickly approved in multiple jurisdictions, so any therapy that aims to be a breakthrough must be developed with an international outlook.
For the time being, CellTrials.org is in a curious position where we are the only cell therapy trials compilation with accurate international data, but we are still working to raise awareness in the cell therapy industry that this matters.
References
- CellTrials.org blog 7 March 2017: Counting Keywords is not Compiling Clinical Trials: Alternative Facts about Cell Therapy and Venture Capital
- CellTrials.org blog 31 Oct. 2017: Counting Keywords is not Compiling Clinical Trials: Placenta
- CellTrials.org blog 31 March 2018:Counting Keywords is not Compiling Clinical Trials: Mesenchymal