Osteoarthritis (OA) is a popular target of regenerative medicine with cell therapy. Since we track all advanced cell therapy at CellTrials.org, we were able to separate the OA trials for the years 2014 through the mid-point of 2019 (June 30), a database of 134 trials. The definition of osteoarthritis is the following: “Sometimes called degenerative joint disease or wear and tear arthritis, osteoarthritis (OA) is the most common chronic condition of the joints. It occurs when the cartilage or cushion between joints breaks down leading to pain, stiffness and swelling.” In this compilation we only included trials that explicitly say their target indication is “osteoarthritis”, and excluded trials that target “cartilage defects”. In this way all the trials are treating degenerative arthritis, and we are not mixing in cartilage damage from sports injuries.
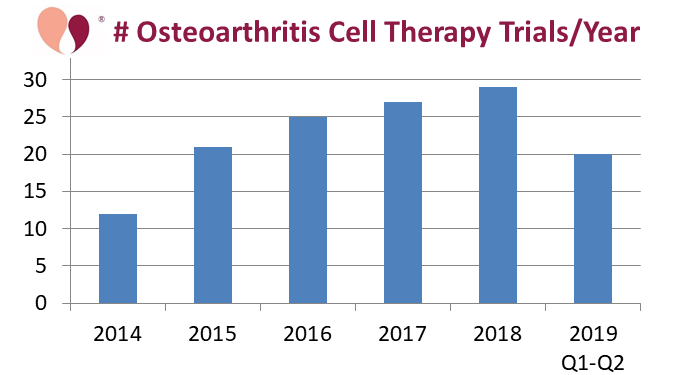
The first figure shows the number of OA trials registered each year, which has been steadily rising. The inspiration for this blog was the observation that so far in 2019 there has been a significant jump in the number of registered trials, an increase of 33% over the first half of last year. In addition, several of the OA trials launched so far in 2019 are late phase and/or enrolling large numbers of patients, as will be described below.
It is complicated to categorize OA trials because there are such a wide variety of cell therapies in trials for this indication. All of the OA trials that state their route of delivery say they are giving injections into the arthritic joint. Some therapies inject suspensions of isolated cells and others are injecting suspensions that contain microfragmented tissue. With this in mind, we present two pie charts that break down the OA trials either by cell therapy source or by cell type.
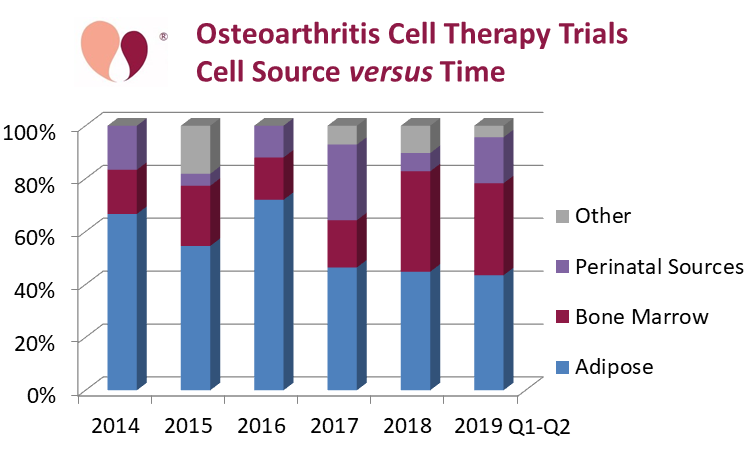
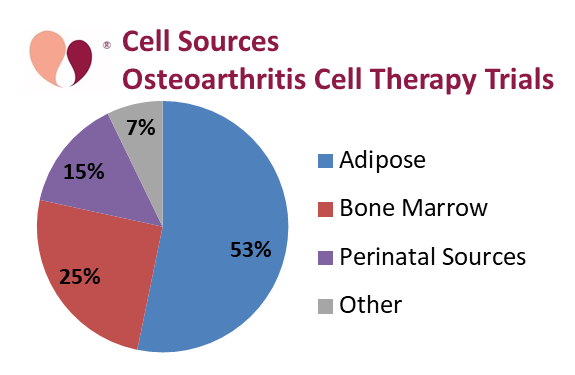 The dominant source material for these OA trials has been adipose tissue, with a total of 53% of the trials, combining 26% that isolate mesenchymal cells from adipose tissue (AT- MSC) and 27% using either stromal vascular fraction (SVF) or microfragmented adipose tissue (MAT). In this graph, those OA trials which had multiple arms using different cell sources were counted towards each source. We call out those sources which appear in at least 10% of the trials. Bone marrow is the source material in 25% of OA trials, which includes 12% using bone marrow aspirate concentrate (BMAC), but there are also smaller numbers of trials that rely on bone marrow mesenchymal cells (BM-MSC), and bone marrow mononuclear cells (BM-MNC). Finally, perinatal sources account for 15% of the OA trials, and this includes 11% from umbilical cord MSC (UC-MSC).
The dominant source material for these OA trials has been adipose tissue, with a total of 53% of the trials, combining 26% that isolate mesenchymal cells from adipose tissue (AT- MSC) and 27% using either stromal vascular fraction (SVF) or microfragmented adipose tissue (MAT). In this graph, those OA trials which had multiple arms using different cell sources were counted towards each source. We call out those sources which appear in at least 10% of the trials. Bone marrow is the source material in 25% of OA trials, which includes 12% using bone marrow aspirate concentrate (BMAC), but there are also smaller numbers of trials that rely on bone marrow mesenchymal cells (BM-MSC), and bone marrow mononuclear cells (BM-MNC). Finally, perinatal sources account for 15% of the OA trials, and this includes 11% from umbilical cord MSC (UC-MSC).
Another way of looking at the source material for OA trials is whether it is autologous, obtained from the patient, or allogeneic, procured from a donor. For example, the therapies based on perinatal sources are always allogeneic. The common therapy sources adipose and bone marrow are always autologous when they are given with limited manipulation (such as SVF or BMAC), because this treatment approach can go from the patient harvest to the delivery of therapy in the same day. By comparison, therapies based on isolated cells are often allogeneic, especially if the cells are expanded or otherwise modified.
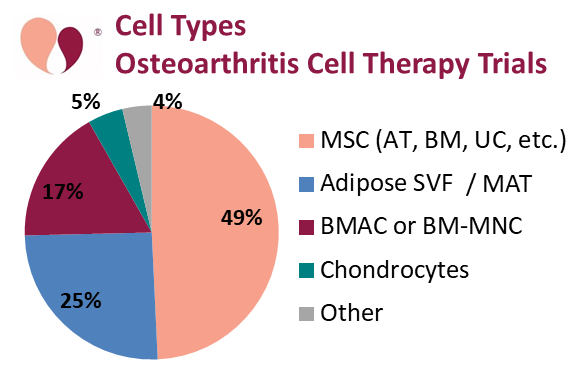 In terms of cell types, 49% of the OA trials rely on MSC isolated from various sources. A small number of trials rely on chondrocytes, a cell type native to cartilage, to repair the damaged cartilage that is the hallmark of OA.
In terms of cell types, 49% of the OA trials rely on MSC isolated from various sources. A small number of trials rely on chondrocytes, a cell type native to cartilage, to repair the damaged cartilage that is the hallmark of OA.
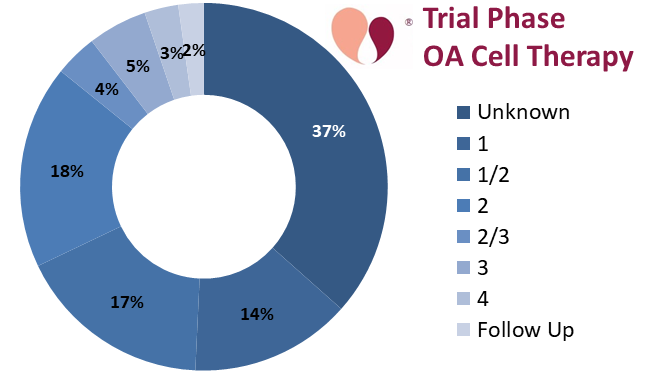
In the remainder of this review we feature particularly notable trials and research groups. We describe all the trials that are phase 3 or higher. Among the 134 OA trials over these 5.5 years, 37% have not specified a phase and only 8% are phase 3 or higher.
The biggest scandal in this arena concerns the Invossa-K injection manufactured by Kolon Life Sciences in South Korea, a licensee of TissueGene in the United States. The Invossa manufacturing process takes allogeneic chondrocytes and genetically modifies them with a viral vector. Invossa-K received market approval in South Korea in July 2017; starting in January 2018 3,000 patients participated in a post-marketing surveillance trial of Invossa-K NCT03412864. But in April 2019 it was reported that Kolon had used cancerous cell lines called GP2-293 cells in their manufacturing process, and they had been “mislabeled” as chondrocytes. The Korean Ministry of Food and Drug Safety quickly suspended the approval of Invossa-K, and launched an investigation to determine why the current drug composition does not match the materials submitted for approval. Not surprisingly, by May 2019 a group of patients who had received Invossa-K filed a class-action lawsuit against the company Kolon TissueGene.
The table below displays a list of cell therapy products that have received market approval for the treatment of OA in major jurisdictions, and are still on the market. A more complete list, which also includes products approved for cartilage repair and products that have been withdrawn, is available from Dr. Alexey Bersenev here.
Table of Cell Therapies with Regulatory Approval to treat Osteoarthritis
| Year | Product | Manufacturer | Cell Type | Country | Approval |
| 2012 | Cartistem® | Medipost | Allo CB-MSC | South Korea | KFDA |
| 2015 | JointStem® | Biostar SCRI | Auto AT-MSC | Japan | Ministry of Health |
| 2016 | (Celution device) | Cytori | Auto MAT | Japan | Act on the Safety of Regenerative Medicine |
| 2016 | (Lipogems device) | Lipogems | Auto MAT | United States | FDA 510(K) |
| 2017 | Chondron | RMS Regrow | Auto Chondrocyte | India | DCGI |
Some of the cell therapy products and devices in this table are currently in clinical trials to expand their base of approvals. The product JointStem®, containing autologous AT-MSC, failed to win a conditional approval in South Korea in 2018 based on its 2015 approval in Japan. Hence in June 2019 JointStem® began a phase 3 trial NCT03990805 that will treat 260 patients with knee OA at 12 hospitals in South Korea.
 The Lipogems technology, which produces “microfragmented” adipose tissue that is claimed to be less manipulated than SVF, already has 510(K) device clearance from the US FDA. This is an interesting situation where there is no named therapy product with an approval, but the approval of the manufacturing device makes it possible to offer adipose therapies for orthopedic indications. Lipogems has registered two phase 4 trials for autologous therapy of knee OA, NCT03117608 in Italy in April 2017 and NCT03922490 in the United States in April 2019.
The Lipogems technology, which produces “microfragmented” adipose tissue that is claimed to be less manipulated than SVF, already has 510(K) device clearance from the US FDA. This is an interesting situation where there is no named therapy product with an approval, but the approval of the manufacturing device makes it possible to offer adipose therapies for orthopedic indications. Lipogems has registered two phase 4 trials for autologous therapy of knee OA, NCT03117608 in Italy in April 2017 and NCT03922490 in the United States in April 2019.
In China, The first NDA/IND approval for a regenerative medicine therapy, under the post-2017 Chinese regulations on cell therapy products, was granted in January 2019 for AlloJoin®, a therapy for knee OA that contains allogeneic AT-MSC. The AlloJoin® product is manufactured by Cellular Biomedicine Group and has been in two clinical trials: NCT02641860 was Phase 1 in 2015 and NCT02855073 was Phase 2 in 2016. The groundbreaking NDA/IND status of this product is not a guarantee of market approval, but it is a necessary pre-condition.
The world’s first phase 3 trial for OA that ran to completion was NCT02726945, sponsored by the GID group in 2016. They used autologous SVF processed by a proprietary device for 39 patients with knee OA. Academic centers are also studying adipose therapies for OA. Stanford University launched a phase 3 trial NCT03467919 in March 2018 to treat 40 patients with knee OA using autologous fat that is “processed with minimal manipulation (Adiprep) in the clinic room.” Another phase 2-3 trial IRCT20151017024572N19 was registered Jan 2019 to give AT-MSC to 30 patients with knee OA at the Iran University of Medical Sciences. The Mayo Clinic is studying autologous fat in two phase 1 OA trials; NCT03608579 from August 2018 treats hip OA with AT-MSC and NCT03940950 from May 2019 treats knee OA with SVF.
The accreditation agency AABB, in partnership with the International Federation for Adipose Therapeutics and Science (IFATS), announced a new accreditation standard for adipose-based therapies in October 2019. “This joint effort focuses on same-day services for the procurement of autologous non-cultured, non-cryopreserved cells and is intended for orthopedics facilities, plastic surgery centers and academic institutions performing same-day, adipose-based therapies.” On the one hand, the establishment of these standards is a sign of how routine adipose therapy has become at the clinic level. But ironically, if you look at the source material in OA clinical trials as a function of time, the adipose share has declined to less than half of the OA trials since 2017.
Bone marrow is also an important source for OA cell therapies in clinical trials. The leading provider in this arena is the Regenexx network of clinics. Their cell therapy utilizes autologous BMAC, and in 2015 they published safety data for a registry of 2372 patients. In January 2017 Regenexx registered a clinical trial NCT03011398 to collect patient registry data for all orthopedic procedures at 32 locations across 3 countries, with a target enrollment of 50,000 patients. Then in April 2019 Regenexx registered trial NCT03898388 to treat 600 patients with knee OA using autologous BMAC at 5 locations in the United States.
Since the start of 2019, four advanced phase clinical trials have launched that employ autologous BMAC. In Serbia, NCT03825133 was retrospectively registered as a phase 4 trial that treated 180 patients with knee OA, comparing BMAC against Platelet Rich Plasma (PRP) or hyaluronic acid. In Italy, NCT03876795 is a phase 3 trial designed to compare the efficacy of BMAC delivery by intra-articular versus intra-osseus routes in 86 patients with knee OA. In Canada, NCT03908827 is a phase 2-3 trial that will enroll 150 patients with knee or hip OA. The fourth phase 3 trial is a multi-arm study described below.
But BMAC is not the only bone marrow therapy that has a late phase OA trial. In India, Stempeutics Research launched a phase 3 trial CTRI/2018/09/015785 in Sept. 2018 of their product Stempeucel®, which contains allogeneic BM-MSC. This trial will enroll 146 patients with knee OA at 16 hospitals in India.
Although we pointed out that perinatal sources account for 15% of the OA trials in this database, the majority of those trials are at academic centers and do not involve a named cell therapy product. One product that has been in trials is ReNu™, which is a cryopreserved allograft composed of particularized amniotic membrane suspended in amniotic fluid. The ReNu™ product was originally developed by NuTech Medical and was acquired by Organogenesis; it has been in trials NCT02318511 in 2014 for 200 patients with knee OA and NCT03063099 in 2017 for 10 patients with hip OA. Another two cryopreserved birth tissue products were in trial NCT03337243 together for 60 patients with knee OA; they are the UC-MSC product CoreCyte™ from Predictive Biotech and the amniotic membrane product SurForce® from Surgenex. In March of 2019, the Medical University of Warsaw launched two parallel trials that will each treat 100 patients with various forms of OA, one trial NCT03869229 using AT-MSC and the other trial NCT03866330 using UC-MSC.
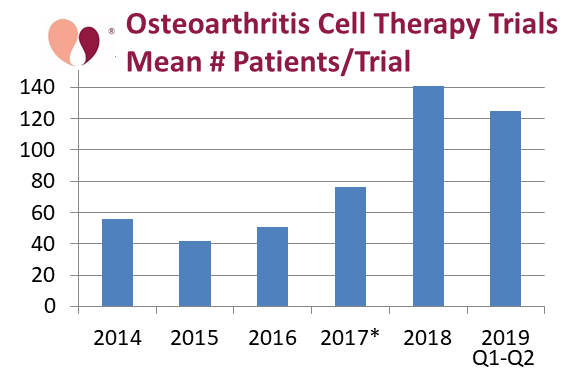 Our last graph shows the average number of patients in the cell therapy trials for OA, by the year in which the trial was first registered. In order to make this graph we omitted the 50,000 patients to be enrolled in the Regenexx patient registry launched in 2017, because it would throw off the statistics and a patient registry is not a pure trial. There is a clear increase in the average size of trials registered in 2018 and 2019.
Our last graph shows the average number of patients in the cell therapy trials for OA, by the year in which the trial was first registered. In order to make this graph we omitted the 50,000 patients to be enrolled in the Regenexx patient registry launched in 2017, because it would throw off the statistics and a patient registry is not a pure trial. There is a clear increase in the average size of trials registered in 2018 and 2019.
The frustrating thing about most of the OA trials is that they test the cell therapy against a very conservative control. The best example of a trial that tests cell therapies against each other is NCT03818737 that launched in January 2019 via collaboration between Emory University and the Marcus Foundation. It is a phase 3 trial for knee OA that has 3 arms: autologous BMAC, autologous SVF, and allogeneic UC-MSC. The trial plans to enroll 480 patients at 5 hospitals in the United States. This Emory/Marcus multicenter trial will use a device from EmCyte to process BMAC, and that device was previously tested in a 2017 phase 4 trial NCT03289416 for 120 patients with knee OA.
In conclusion, the use of cell therapy to treat OA, especially knee OA, is clearly a broad and active area of research, with products that are already approved and others that seem to be near approval.