Frances Verter, PhD
In our last blog we pointed out that there was 2% growth from 2018 to 2019 in the total number of trials registered worldwide performing advanced cell therapy, which includes therapy with both cells and gene-modified cells. In our follow-up blogs, we will look at the contribution of specific cell types to the overall trends. Despite the overall slow growth, certain new therapies are breaking out and growing rapidly.
Our first graph is a timeline of the number of trials per year that employ CAR-construct immunotherapy (CAR is an abbreviation for Chimeric Antigen Receptors) versus the number of trials that employ mesenchymal stromal cells (MSC). This comparison pits the favorite immunotherapy cell type against the favorite regenerative medicine cell type. While the number of MSC trials per year has grown by half again over the past decade, the number of CAR trials has grown 25-fold over the same time!
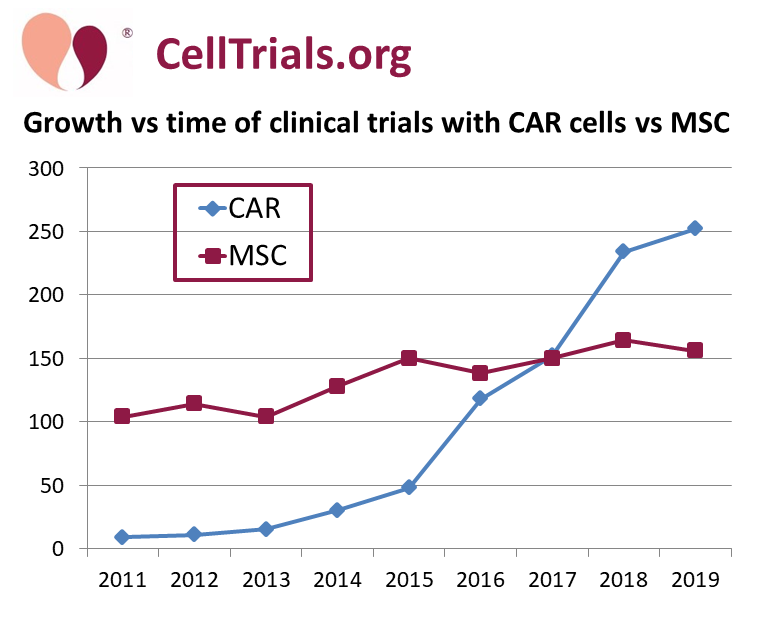
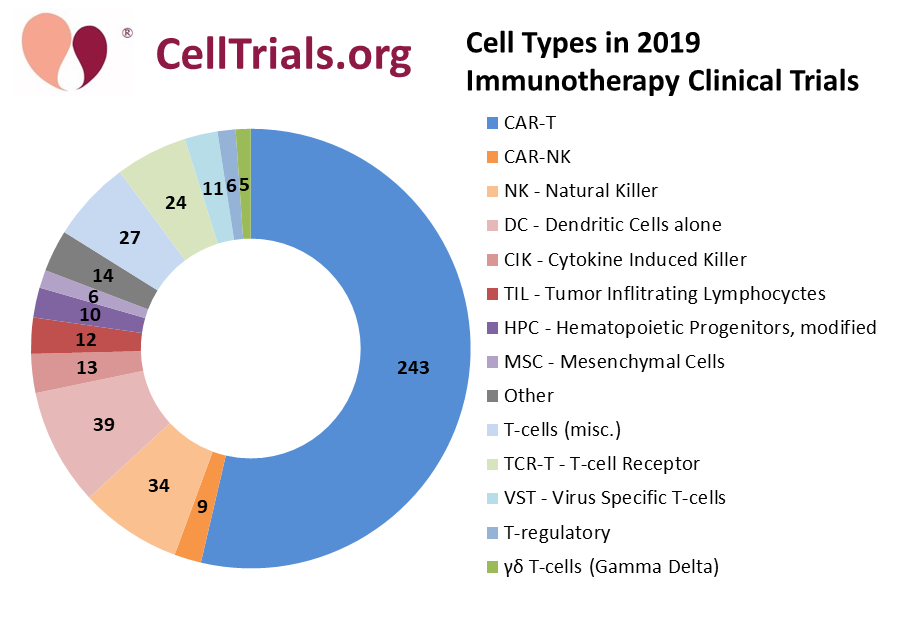
In 2019, CellTrials.org counted 453 immunotherapy trials worldwide, out of 761 newly registered trials. The fraction of new trials only listed on registries outside of ClinicalTrials.gov was 31%. Almost 60% of the 2019 advanced cell therapy trials were immunotherapy. These trials utilized a wide variety of expanded or engineered cell types, as shown in the ring diagram below.
The most popular immunotherapy by far is CAR-T, covering 54% of the immunotherapy trials. Also, if you combine the various slices in shades of blue or green that employ some type of T-cell, the T-cell trials add up to 70% of all immunotherapy clinical trials. Those readers who attended Dr. Verter’s talk at Phacilitate 2020 will recognize this picture as the one where she fell off the stage.
In coming blogs we intend to discuss: the shift towards CAR-T trials with allogeneic cells, a comparison of CAR versus NK trial growth, the emergence of gene therapy with HPC for inherited disorders, and trends in MSC trials.