The most surprising news about the state of cell and gene therapy at the end of 2019 is that there was little growth in the total number of advanced cell therapy trials registered worldwide from 2018 to 2019. These include clinical trials of cell therapy and trials with gene-modified cells. This insight is only available from CellTrials.org, because we track 16 clinical trial registries around the world.
The histogram below shows the total number of advanced cell therapy trials registered each year. In most years, the fraction of advanced cell therapy trials "outside" ClinicalTrials.gov is between 20% and 30%; for example in 2019 it was 31%. However 2018 was an unusual year in which the outside fraction was 38%. As a result, tracking ClinicalTrials.gov alone would yield the impression of 14% growth from 2018 to 2019, when in fact there was only 2% growth in the total number of advanced cell therapy trials worldwide (total trials registered were 743 in 2018 and 761 in 2019).
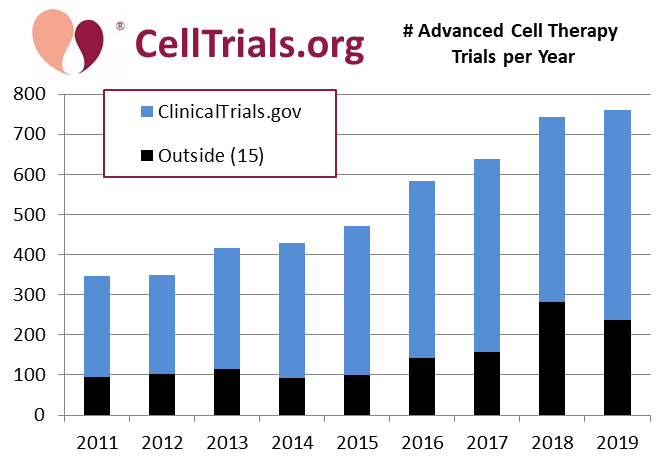
We interpret this stabilization of growth as a sign that the field of cell and gene therapy is maturing. We would not characterize the field as stalled, given that there are about 750 new trials per year. The emphasis now is on pushing towards later phase trials and approved therapies.
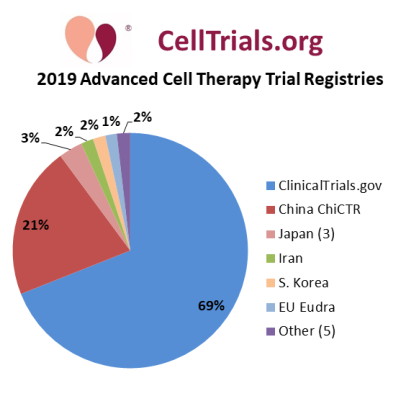 In a previous blog we provided a table of the registries from which we compile trials, and the first pie chart shows their contributions towards the total 2019 clinical trials in advanced cell therapy. The majority of the trials registered in 2019, 69%, are listed on ClinicalTrials.gov. But 31% of the 2019 trials were found on 12 other registries (out of 16 searched), especially 21% in China, and these contributions would be overlooked without a comprehensive search of all national trial registries. In our database, we eliminate cross-posting of the same trial to multiple registries, and assign it to the registry where it was listed first, or to ClinicalTrials.gov if two registrations were within the same month.
In a previous blog we provided a table of the registries from which we compile trials, and the first pie chart shows their contributions towards the total 2019 clinical trials in advanced cell therapy. The majority of the trials registered in 2019, 69%, are listed on ClinicalTrials.gov. But 31% of the 2019 trials were found on 12 other registries (out of 16 searched), especially 21% in China, and these contributions would be overlooked without a comprehensive search of all national trial registries. In our database, we eliminate cross-posting of the same trial to multiple registries, and assign it to the registry where it was listed first, or to ClinicalTrials.gov if two registrations were within the same month.
For more background on cell and gene therapy clinical trials in China, we reference our previous blog on this topic, which cites a recent publication explaining the current cell therapy regulations in China. A one sentence overview is that the Chinese registry ChiCTR is intended to list clinical trials that are on track towards market approval. Therefore, even though the sequence of steps towards market approval in China differs from Western regulations, it is accurate to characterize trials on ChiCTR as comparable to and competing with advanced cell therapy in other trial registries.
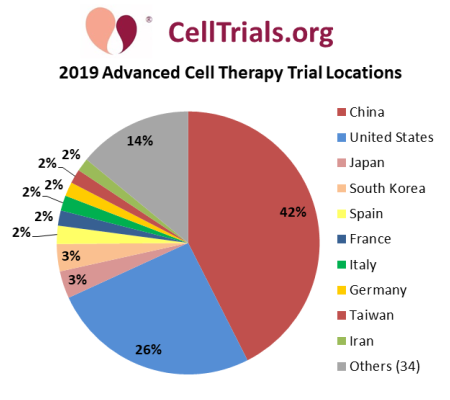 The second pie chart displays the countries in which the 2019 advanced cell therapy trials are being or will be conducted. The 761 trials will recruit in 44 countries, with a dozen of the trials (1.6%) designed as multi-national. Only 10 countries will host 2% or more clinical trials, and the biggest fraction is 42% in China. Notice that the fraction of trials taking place in China, 42%, exceeds the fraction of trials that are registered on the Chinese registry ChiCTR, 21%: this is because trials being conducted in China may be registered on either ClinicalTrials.gov or ChiCTR. While most of the world’s advanced cell therapy trials, 69%, are registered on ClinicalTrials.gov, only 26% of trials will be conducted in the United States.
The second pie chart displays the countries in which the 2019 advanced cell therapy trials are being or will be conducted. The 761 trials will recruit in 44 countries, with a dozen of the trials (1.6%) designed as multi-national. Only 10 countries will host 2% or more clinical trials, and the biggest fraction is 42% in China. Notice that the fraction of trials taking place in China, 42%, exceeds the fraction of trials that are registered on the Chinese registry ChiCTR, 21%: this is because trials being conducted in China may be registered on either ClinicalTrials.gov or ChiCTR. While most of the world’s advanced cell therapy trials, 69%, are registered on ClinicalTrials.gov, only 26% of trials will be conducted in the United States.
In our next blog, we will go beyond the total numbers of 2019 clinical trials and look for emerging trends in advanced cell therapy.
2019 Overview of Advanced Cell Therapy Clinical Trials
I would challenge the comment that this industry is maturing!!