Authors: Frances Verter & Pedro Silva Couto
During spring 2020, CellTrials.org launched a public service of compiling and sharing detailed data packages that listed and described all clinical trials of COVID-19 interventions registered worldwide. At CellTrials.org, when we compile trials worldwide, we search over a dozen national registries, as described in previous publications about our work.
Our first graph shows all interventional trials for COVID-19, from the beginning of 2020 through the end of April, binned by number per week and color coded by the country from which the trial was registered. Each week runs Monday to Sunday and is labeled by the 7th day. During this time frame we collected 938 COVID-19 interventional clinical trials. Each trial is counted only once and assigned to the country where it was registered, even if it intended to recruit patients in multiple countries.
The novel coronavirus COVID-19 emerged in China sometime in late 2019, but it was not recognized as a serious threat until the very end of that year, and the first COVID-19 clinical trials were registered in China during the last week of January 2020. During the month of February 2020 (the exact days Feb 1 to 28), 97% of interventional trials for COVID-19 were registered by entities in China, and listed on either the Chinese trial registry ChiCTR or the United States registry ClinicalTrials.gov.
During the period 1-31 March 2020, COVID-19 epicenters emerged in the United States, in Iran, and in several European nations. Not surprisingly, COVID-19 clinical trials registrations also surged in these locations. New trials in China dropped to 51% of registrations.
By the end of April, tracking COVID-19 clinical trials by country had become very complicated. Over the period 1-30 April 2020, new trial registrations were 7% China, 32% United States, 11% France, 7% Spain, 5% Iran, 5% Italy, and the remaining 32% of trials were spread across 45 countries.
Even though our timeline only covers three months, it clearly shows that the launch of clinical trials in China dropped dramatically once the outbreak in that country had been largely contained. Although most of the trials registered in China have not been officially withdrawn, one has to speculate if they are still recruiting? One well-publicized example was the announcement that the Chinese trial of the Gilead drug Remdesivir was closed early due to lack of eligible patients.
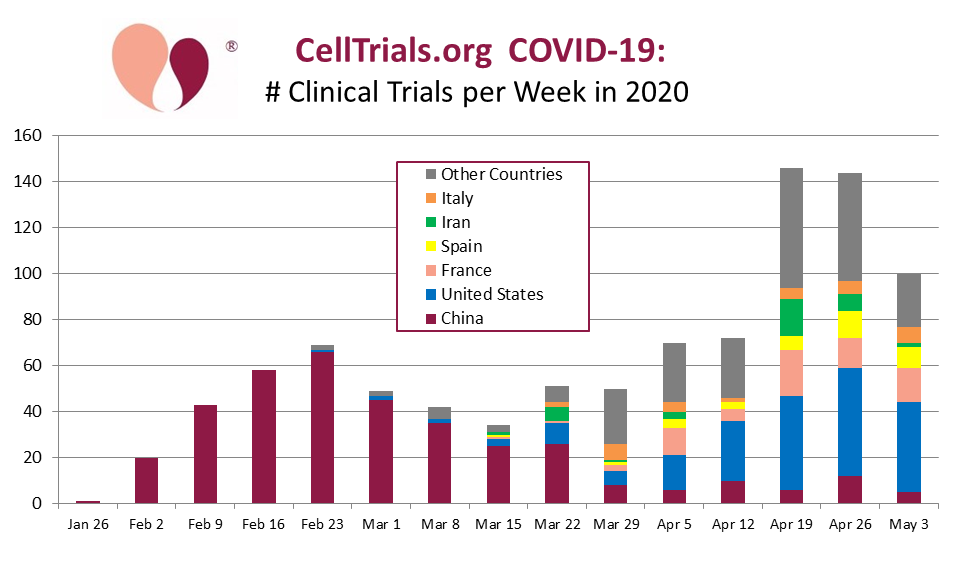
The majority of these trials, 62%, were registered or cross-posted to ClinicalTrials.gov, but an additional 31% of trials were only listed on the Chinese registry ChiCTR, 3% on the European registry EudraCT, 3% on the Iranian registry IRCT, and the remainder on other registries in Australia, Japan, etc.
In summary, we believe this graph serves to illustrate that COVID-19 is a moving target for clinical trials. As the epicenters of COVID-19 outbreaks move, either from one country to another or within a country, clinical trial managers must partner with different hospitals to recruit patients. Only those trial sponsors that are strong enough to form relationships with multiple hospitals will be able to accrue large numbers of patients for their trials.
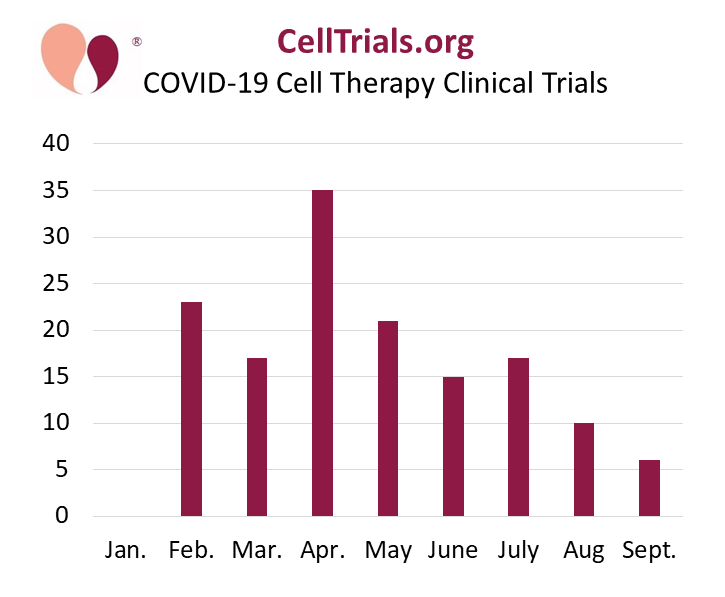
At CellTrials.org, we have stopped compiling all clinical trials for COVID-19 and now provide a FREE spreadheet of cell therapy clinical trials for COVID-19, our core expertise. For the first half of 2020, we listed 111 COVID-19 cell therapy trials registered worldwide. In practice that covers February to June, because there were no cell therapy COVID-19 trials in January.
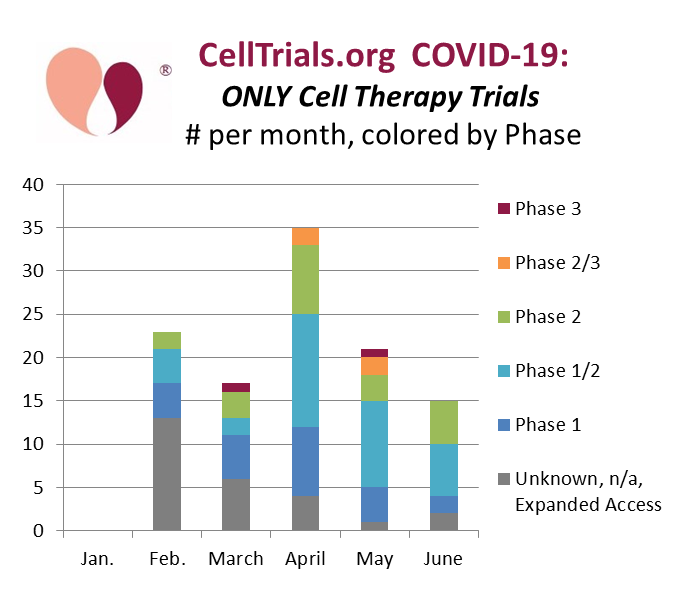
Our second graph shows all cell therapy clinical trials for COVID-19, binned by number per month and color coded by trial phase. It is not surprising that with the passage of time more trials of later phase are launching. It is notable that the total number of new trials is declining despite the fact that COVID-19 hospitalizations continue unabated in the United States. The decline of new trial registrations suggests to us that those companies that were willing and able to compete in cell therapy for COVID-19 have already entered the market.
This update showing trial numbers for the first three quarters of 2020 confirms our view that most cell therapy companies willing and able to apply their products to COVID-19 have already launched clinical trials.