Package of all MSC clinical trials registered on 15 national registries from 2011 through 2020 contains at least 15 parameters for 1499 trials. Only trials with isolated MSC are included, from any source. This package is an accurate list of mesenchymal cell trials regardless of whether the mesenchymal cells are considered to be stem cells, stromal cells, signaling cells, etc. We exclude trials where the mechanism of action comes from exosomes released by MSC; we have a separate data product for exosome trials.
- Trial Data
- Registration Year
- Trial ID with link to trial on-line
- Country
- Phase
- Status (at time of registration)
- Cell Data
- Cell Type
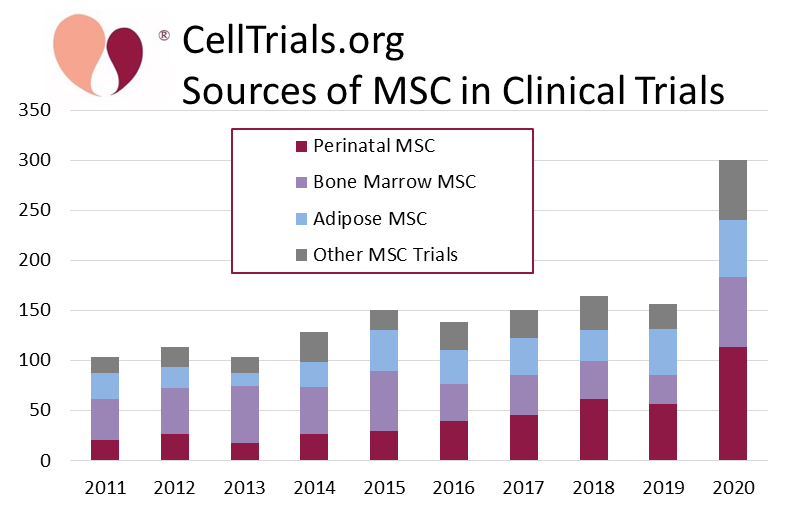
- Cell Source
- Route of Administration
- Cell Dose
- Patient Data
- Indication for use
- Autologous or Allogeneic Therapy
- Target Enrollment
- Patient Age Groups
- Sponsor Data
- Academia or Industry Funding
- Sponsor Names - Only industry names before 2017, since 2017 all sponsors and collaborators are included
- Product Name (if any) - included since 2017
| Attachment | Size |
|---|---|
| Sample MSC Trials 2011-2020 | 20.48 KB |