I’m Pedro Silva Couto, and this blog combines content from my personal blog, Cell and Gene Therapy Today, together with data from my membership on the team at CellTrials.org.
Recent clinical trials of note within the cell and gene therapy space
 NCT06716281 was registered by the Chinese company Shanghai ixCell Biotechnology Co. Ltd. The company has a pipeline of human mesenchymal stromal cells (hMSC) products for stroke and pulmonary fibrosis and has recently entered the immunotherapy space by developing product candidates for autoimmune diseases and solid tumours using iPSC-NK and other undisclosed immune cells. This new study is a phase 3, randomised, double-blind placebo controlled clinical trial of the company's lead product, umbilical cord tissue human mesenchymal stromal cells (UC-hMSCs) for knee osteoarthritis. In this study, the sponsor aims to recruit close to 400 participants who will receive a single intra-articular injection at a fixed dose of 5x107 UC-hMSCs. In the trial registration, the investigators have alluded to the known apoptotic, anti-fibrotic, anti-inflammatory and pro-angiogenic properties of UC-hMSCs, which are the drivers for this candidate's expected clinical efficacy.
NCT06716281 was registered by the Chinese company Shanghai ixCell Biotechnology Co. Ltd. The company has a pipeline of human mesenchymal stromal cells (hMSC) products for stroke and pulmonary fibrosis and has recently entered the immunotherapy space by developing product candidates for autoimmune diseases and solid tumours using iPSC-NK and other undisclosed immune cells. This new study is a phase 3, randomised, double-blind placebo controlled clinical trial of the company's lead product, umbilical cord tissue human mesenchymal stromal cells (UC-hMSCs) for knee osteoarthritis. In this study, the sponsor aims to recruit close to 400 participants who will receive a single intra-articular injection at a fixed dose of 5x107 UC-hMSCs. In the trial registration, the investigators have alluded to the known apoptotic, anti-fibrotic, anti-inflammatory and pro-angiogenic properties of UC-hMSCs, which are the drivers for this candidate's expected clinical efficacy.  NCT06720324 was registered by Wuhan Union Hospital Medical College in China to take advantage of a novel CAR-based approach: endogenous CD7 in T-cells causes CD7-targeting CAR-T cells to kill each other in vivo. This phenomenon described as fratricide in the CAR-T cell therapy field. The sponsors will evaluate the safety and efficacy of an anti-CD7 CAR-T cell product where the endogenous CD7 receptor expression has been stopped (suggesting a gene-silencing-based approach rather than a knockout strategy). Although there is no clear explanation regarding the manufacturing process for this therapy, the clinical trial registration mentions the usage of a 2nd generation CAR construct delivered in a lentiviral vector designed for CD7 targeting. This phase 1/2, single group, open label clinical trial is expected to enrol 80 patients suffering from haematological malignancies. This approach has been implemented in other clinical trials targeting receptors such as CD5.
NCT06720324 was registered by Wuhan Union Hospital Medical College in China to take advantage of a novel CAR-based approach: endogenous CD7 in T-cells causes CD7-targeting CAR-T cells to kill each other in vivo. This phenomenon described as fratricide in the CAR-T cell therapy field. The sponsors will evaluate the safety and efficacy of an anti-CD7 CAR-T cell product where the endogenous CD7 receptor expression has been stopped (suggesting a gene-silencing-based approach rather than a knockout strategy). Although there is no clear explanation regarding the manufacturing process for this therapy, the clinical trial registration mentions the usage of a 2nd generation CAR construct delivered in a lentiviral vector designed for CD7 targeting. This phase 1/2, single group, open label clinical trial is expected to enrol 80 patients suffering from haematological malignancies. This approach has been implemented in other clinical trials targeting receptors such as CD5.  NCT06713902 was registered by the I.R.C.C.S Ospedale Galeazzi-Sant'Ambrogio of Italy and aims to study the feasibility of using hMSCs delivered on a platelet-rich plasma-derived fibrin gel in patients suffering from joint injuries. The proposed mechanism of action is based on the idea that the PRP will provide growth factors, and the extracellular vesicles from MSC (hMSC-EVs) will be responsible for the cartilage-stimulating activity. The product uses adipose tissue as an hMSC source and autologous blood as PRP source. A previous publication describes the manufacturing process. This observational study aims to recruit over 50 participants to research the product profile and its biological feasibility in an interventional clinical trial.
NCT06713902 was registered by the I.R.C.C.S Ospedale Galeazzi-Sant'Ambrogio of Italy and aims to study the feasibility of using hMSCs delivered on a platelet-rich plasma-derived fibrin gel in patients suffering from joint injuries. The proposed mechanism of action is based on the idea that the PRP will provide growth factors, and the extracellular vesicles from MSC (hMSC-EVs) will be responsible for the cartilage-stimulating activity. The product uses adipose tissue as an hMSC source and autologous blood as PRP source. A previous publication describes the manufacturing process. This observational study aims to recruit over 50 participants to research the product profile and its biological feasibility in an interventional clinical trial.
Suspension-Adapted hMSC
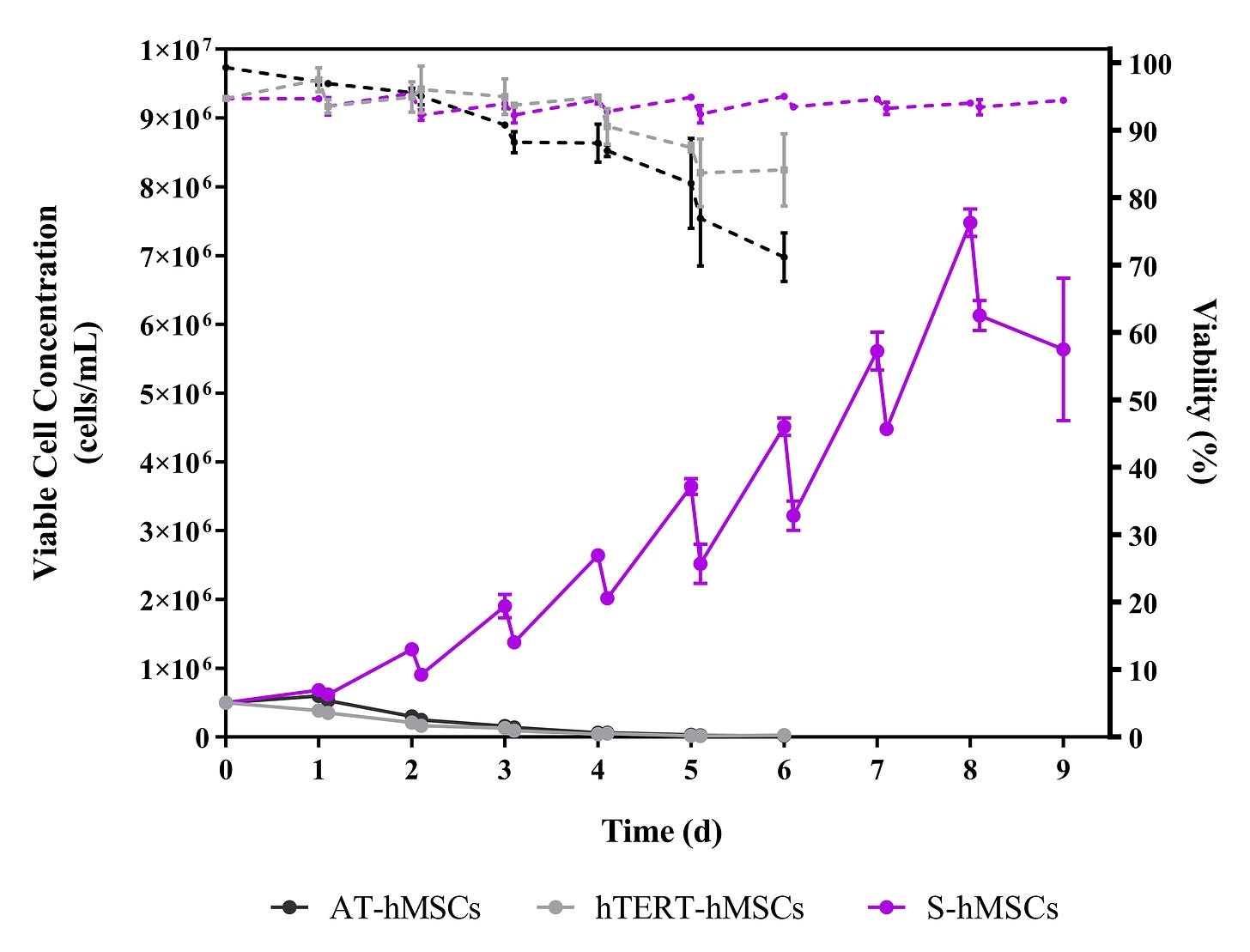
A breakthrough in human mesenchymal stromal cell (hMSC) culture was made by a research team at UCL. They are the first ever to develop a method to grow immortalised hMSC in suspension, without relying on cell adherence to a carrier. These advancements make it possible to manufacture extracellular vesicles (EV, also known as exosomes) at scale, and thereby will accelerate the clinical applications of EV. It is anticipated that suspension-adapted hMSC will have a transformative impact on the MSC and EV bioprocessing fields, similar to the revolutionary effect that suspension-adapted CHO (Chinese hamster ovary) cells had on the production of monoclonal antibodies.
Citation:
Silva Couto P, Stibbs DJ, Sanchez BC, Khalife R, Panagopoulou TI, Barnes B, George V, Taghizadeh RR, Rafiq QA.
Generating suspension-adapted human mesenchymal stromal cells (S-hMSCs) for the scalable manufacture of extracellular vesicles.
Cytotherapy. December 2024; 26(12):1532-1546.