Authors: Pedro Silva Couto & Frances Verter
After publishing our first blog announcing the launch of our exosome clinical trials database, we now explain the definitions and criteria that we used to select clinical trials with extracellular vesicles (EVs) and/or conditioned medium (CdM).
An important reminder is that the International Society for Extracellular Vesicles (ISEV) defines “extracellular vesicles” as the “particles naturally released from the cell that are delimited by a lipid bilayer and cannot replicate, i.e. do not contain a functional nucleus”1. With this definition in mind we only include clinical trials that use either EVs or CdM that are known to be released from a cell. We also require that there is a manufacturing process designed to isolate and purify the final EV or CdM product.
To illustrate how these inclusion/exclusion criteria work, here are some examples: We include EV/CdM that are isolated from mesenchymal stromal cells (MSC). We include clinical trials that use EV which are isolated from tumor cells that are packaged with methotrexate. On the other side, we exclude EVs released from tissues during cell isolation, because in this situation it is not possible to associate the EV with a specific cell type, plus the EVs did not undergo a purification process. We exclude all “blood EV” from plasma, partly because platelet-rich plasma (PRP) is not a cellular therapy, and also because particles or medium derived from plasma are not a specific EV population that has been isolated through a manufacturing process.
Below we list the keywords that we used to search for EV clinical trials, and the number of trials that we found before we reviewed each trial to select only EV/CdM manufactured from cells. An important caveat is that we search all clinical trial registries worldwide, not just ClinicalTrials.gov. We have included the national clinical trial registries in China, the EU, Iran, Japan, the WHO, etc. We use this approach for all our data products and it has been documented in our publications2.
Keyword Search | Results Obtained (before analysis) |
apoptotic bodies | 11 |
cell free | 479 |
conditioned medium | 40 |
culture medium | 299 |
ectosome | 379 |
exosome | 11 |
expansion medium | 226 |
microparticle | 273 |
oncosome | 7 |
paracrine | 91 |
prostasome | 3 |
secretome | 43 |
supernatant | 227 |
vesicles | 752 |
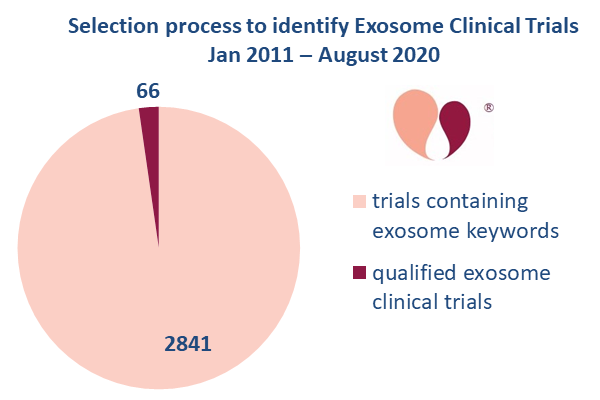
At present, from January 2011 through August 2020, we have found 66 clinical trials worldwide that meet our definition of an EV/CdM clinical trial. Note that only 2% of the trials found by keyword searches were qualified as an “exosome trial” after applying the requirements that the exosome product must come from cells and must go through a manufacturing process. There are certainly clinics selling “exosome products” to patients that do not meet these criteria. But without a manufacturing process that purifies and clearly characterizes the contents of the product, these “exosomes” cannot pass regulatory approval3.
Here is our take-home message: A casual search of ClinicalTrials.gov using the keyword “vesicles” will lead to the impression that there are hundreds of clinical trials with EV. That is a big mistake. There are not even 100 clinical trials registered yet in the whole world that fully meet the ISEV definition of extracellular vesicles for therapeutic application. If you read blogs or papers by other authors who claim to have more clinical trials than us, check to see how carefully they reviewed those trials to apply the inclusion/exclusion criteria that we have explained here. These criteria are essential to generate an accurate and reproducible database of EV products in clinical trials.
References
- 1. Théry C, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. 3. FDA. Consumer Alert on Regenerative Medicine Products Including Stem Cells and Exosomes. Journal of Extracellular Vesicles 2018; 7(1):1535750.
- 2. Couto PS, Bersenev A, Verter F. The first decade of advanced cell therapy clinical trials using perinatal cells (2005–2015). Regenerative Medicine 2017; 12(8):953-968.
- 3. FDA. Consumer Alert on Regenerative Medicine Products Including Stem Cells and Exosomes. Published 2020-07-22