Dale Stibbs, Pedro Silva Couto, and Frances Verter
The newest database from CellTrials.org, released April 2022, holds 879 clinical trials registered worldwide 2011-2021 in which gene therapy is given in vivo, without cells as carriers. This is a complement to our other data products that track advanced cell therapy, including cells that have genetic modifications, such as CAR-T. During in vivo gene therapy, the gene modification takes place inside the patient’s body, rather than modifying cells outside the body and then giving the cells to the patient. Typically, a virus is used as the vector that delivers the in vivo gene therapy, although we also track non-viral delivery methods such as plasmid DNA. This database does not include vaccine clinical trials, because vaccines are not intended to change the patient’s genetics and therefore are not gene therapy.
Readers who work in gene therapy are no doubt aware of the on-line database from the Journal of Gene Medicine, which holds all gene therapy clinical trials worldwide, compiled from various registries and publications. As of this writing, that website shows statistics for 3180 cumulative clinical trials. It is important to note that their resource is a mixture of gene therapy in cells and gene therapy in vivo. A review article is published about the J Gene Medicine database every few years, with the most recent paper covering data through 20171. Our database is different because we offer the ability to download a full list of the gene therapy trials, which is not possible with the J Gene Medicine website.
Our in vivo gene therapy database is a searchable and sortable list of 15 parameters for 879 trials. The trials were collected with uniform search criteria, throughout a uniform time period, consistent with academic standards. A total of 66,867 trials were searched to find the 879 trials of in vivo gene therapy from 2011 to 2021. As with other products from CellTrials.org, clinical trials were collated from multiple national registries, including: United States (ClinicalTrials.gov), Australia, China, European Union, Iran, Japan, South Korea, United Kingdom, and the WHO trial aggregator. Each trial was read by a human scientist; we did not rely on software that picks up keywords without context and generates numerous false positives. The 29 keywords searched for this compilation were: AAV, Adenoviral vector, Adenovirus, Alphavirus, Coxsackievirus, Designer, DNA, Edited, Engineered, Gene, HSV, Lentiviral vector, Lentivirus, Maraba virus, Measles virus, naked DNA, Newcastle disease virus, Oncolytic virus, Parvovirus, Plasmid, Poliovirus, Pox virus, Reovirus, Retrovirus, Rhinovirus, Sendai Virus, Vaccinia, Vector, Vesicular Stomatitis Virus.
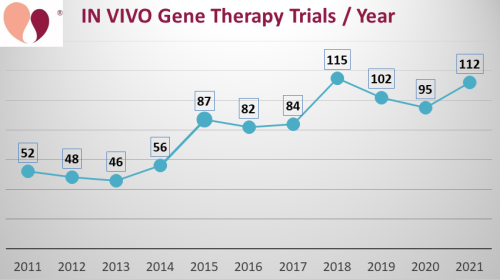
The first graph shows the number of clinical trials per year performing in vivo gene therapy. We believe this is the first time anyone has published a timeline of in vivo gene therapy alone. The number of trials per year has roughly doubled from 2011 to 2021 (from 52 to 112 trials). This is comparable to the growth of the overall field of advanced cell therapy during those years seen in the database of CellTrials.org (from 346 to 773 trials). Whereas, the number of CAR-immunotherapy trials increased 29 -fold from 2011 to 2021 (from 9 to 262 trials).
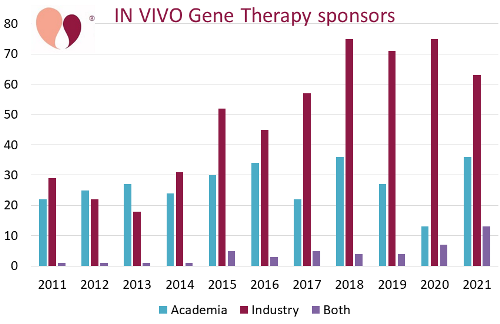
The second graph displays the source of funding for in vivo studies of gene therapy over the years from 2011 to 2021. To our knowledge, none of the published review articles on gene therapy have tracked trial sponsorship as a function of time. Whereas initially the clinical trials were split nearly in half between academic versus industry funding, over the past decade the clinical research of in vivo gene therapy has come to be dominated by corporate sponsorship.
The field of in vivo gene therapy research has struggled to achieve approved and successful commercial products2-4. We plan to write another blog that will go into more detail about the diversification of delivery vectors being used by in vivo gene therapy clinical trials.
References
- Ginn SL, Amaya AK, Alexander IE, Edelstein M, Abedi MR. Gene therapy clinical trials worldwide to 2017: An update. Journal of Gene Medicine 2018; 20(5):e3015
- Keeler AM and Flotte TR. Recombinant Adeno-Associated Virus Gene Therapy in Light of Luxturna (and Zolgensma and Glybera): Where Are We, and How Did We Get Here? Annual Review of Virology 2019; 6:601-621
- Zhao Z, Anselmo AC, Mitragotri S. Viral vector-based gene therapies in the clinic. Bioengineering &Transl. Med. 2022; 7(1):e10528
- Pagliarulo N. As biotech retreats, gene therapy companies retrench and redraw plans. BiopharmaDive Published 2022-03-24