Authors: Pedro Silva Couto & Frances Verter
CellTrials.org has just launched the world’s first database of exosome clinical trials and their publications. Although we use the popularized term exosomes to title our database and this blog, in fact we include all clinical trials that employ any form of Extracellular Vesicles (EV) and/or Conditioned Medium (CdM). Below we explain the rationale for these selection criteria.
According to the International Society for Extracellular Vesicles (ISEV), “extracellular vesicles” is the term used to describe “particles naturally released from the cell that are delimited by a lipid bilayer and cannot replicate, i.e. do not contain a functional nucleus”1. Table 1 summarizes several types of small vesicles that may be released by cells (adapted from György et al. 2). Only one of these EV types is technically called “exosomes”.
Type of Vesicle | Size | Mechanism of generation |
Exosome | 30-100 nm | Exocytosis of multivesicular bodies |
Microvesicles | 100 nm to 1 µm | Budding off the plasma membrane |
Apoptotic Body | 1 to 5 µm | Released from cells during apoptosis |
In recent years there has been growing interest in the therapeutic potential of EVs. A few of the biological properties that make EV suitable carriers to deliver therapies are listed:
- EVs are naturally used for cell-cell communication
- EVs can be used as molecule carriers
- EVs can be isolated from gene-modified cells
- EVs can cross the blood-brain barrier (unlike cells)
Some research groups have demonstrated in pre-clinical studies that EVs which are isolated from gene-modified cells and/or loaded with chemotherapy drugs are effective at eliminating cancer cells3,4.
Experimentally, it is challenging to identify and quantify different types of EV. In order to legitimately claim that a product contains EVs, the manufacturing process must include procedures to enrich and/or isolate EVs. In practice, many authors use the terminology “EVs” or “Exosomes”, when in reality they are only using a form of conditioned medium1. Because it is impossible to police the authors of clinical trials and papers to make sure that all claims about EV are valid, we have chosen to include clinical trials with both extracellular vesicles (EV) and conditioned medium (CdM) in our database.
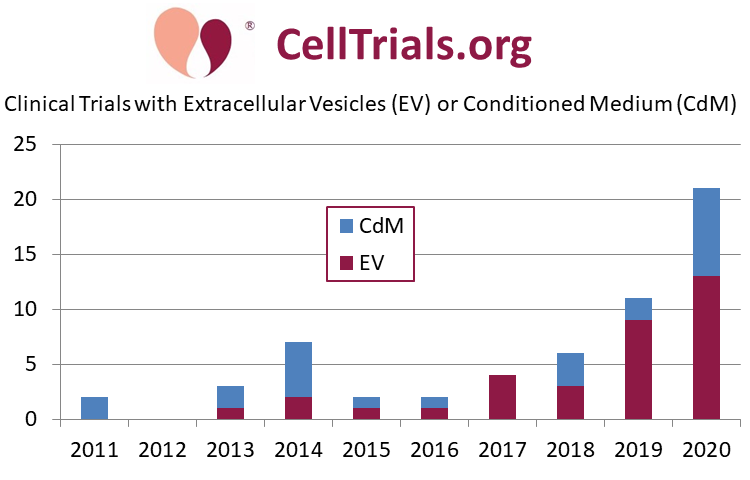
The first CellTrials.org Exosomes Database contains 58 clinical trials registered worldwide between January 2011 and June 2020. The contents of our database are described here. We consider 34 of these trials to be utilizing EVs and 24 to be based on CdM. Figure 1 displays the number of trials registered per year, clearly capturing the recent increased use of this therapeutic modality (especially considering that the data for 2020 is a half year!). To date, none of these clinical trials have reported their results in a publication.
Within the United States, many clinics are marketing “exosome therapies” to consumers on the premise that, since they do not contain cells, they are not subject to federal regulation or safety concerns. This is false advertising. In fact, the US FDA regulates EVs on the same pathway as advanced cell therapy – i.e. they are treated like drugs5.
We hope to produce more blogs about “exosomes” in the months ahead to discuss additional aspects of this heterogeneous but rapidly growing field.
References
- 1. Théry C, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles 2018; 7(1):1535750.
- 2. György B, Szabó TG, Pásztói M, et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cellular and Molecular Life Sciences 2011; 68:2667–2688.
- 3. Yuan ZQ, Kolluri KK, Gowers KHC, and Janes SM. TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy. Journal of Extracellular Vesicles 2017; 6(1):1265291.
- 4. Ye Z, Zhang T, He W, et al. Methotrexate-Loaded Extracellular Vesicles Functionalized with Therapeutic and Targeted Peptides for the Treatment of Glioblastoma Multiforme. ACS Applied Materials & Interfaces 2018; 10(15):12341–12350.
- 5. FDA. Consumer Alert on Regenerative Medicine Products Including Stem Cells and Exosomes. Published 2020-07-22