Several reports have come out indicating that the coronavirus pandemic is having a devastating impact on clinical trials in general (see additional references at the end of the linked article). Hundreds of oncology trials have been suspended, and ongoing trials in all fields are struggling to monitor patients.
Nonetheless, CellTrials.org reports that during the first quarter of 2020, the rate at which new trials have been registered in the field of advanced cell therapy is almost 30% higher than the averages for the years 2019 and 2018. Specifically, the mean registration rate during the first quarter of 2020 is 81 trials per month, whereas the annual averages for 2019 and 2018 were 63 and 62 trials per month, respectively. Bear in mind these are strictly trials in advanced cell therapy, and we include all national registries. Below we display the number of new trials each month, because in a typical year there is a lot of month to month variation in trial registrations.
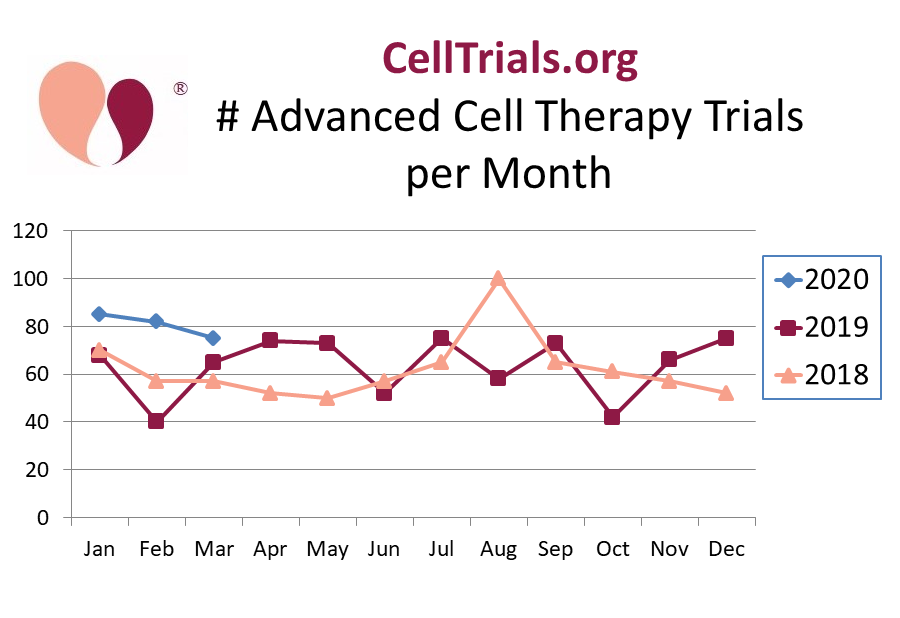
Is the increase in new trial registrations simply due to a flood of new trials using cell therapy to treat COVID-19? No, it is not. We looked at the fraction of cell therapy trials that explicitly or implicitly target COVID-19. Under the “implicit” category we included trials for pneumonia or ARDS that did not explicitly say they are treating COVID-19 patients. We found the COVID-19 fraction of international advanced cell therapy trials was 0% in January, 28% for February, and 21% in March. For the first quarter of 2020 as a whole, removing the COVID-19 trials would reduce cell therapy registrations to 68 trials per month, which is similar to the previous two years.
But, it is remarkable that non-COVID-19 advanced cell therapy trials continue to be registered at previous levels!! We looked at several different aspects of the non-COVID-19 trials from 2020 Q1 to see if they differed from 2019. There were no obvious flags. For example, the breakdown by dominant countries is roughly the same: During 2019, 45% of advanced cell therapy trials were in China and 27% in the USA, whereas in 2020 Q1 the non-COVID-19 cell therapy trials are 41% China and 25% USA. Another example, the fraction of these trials performing immunotherapy is 60% for both 2019 and 2020 Q1.
There is the possibility that new cell therapy trials are being registered because researchers have time to file the paperwork, but these trials are not actually recruiting patients. Among the non-COVID-19 cell therapy trials registered this past quarter, 47% claim to be active. During all of 2019, 50% of cell therapy trials were active when they first registered.
We are surprised by the robust number of advanced cell therapy trials that have been registered so far this year. It is only three months of data, so we will continue to monitor and see if this rate of registrations continues, and if any correlation emerges that helps to explain trends in the data.