Frances Verter PhD, Pedro Silva Couto MSc, Alexey Bersenev MD PhD
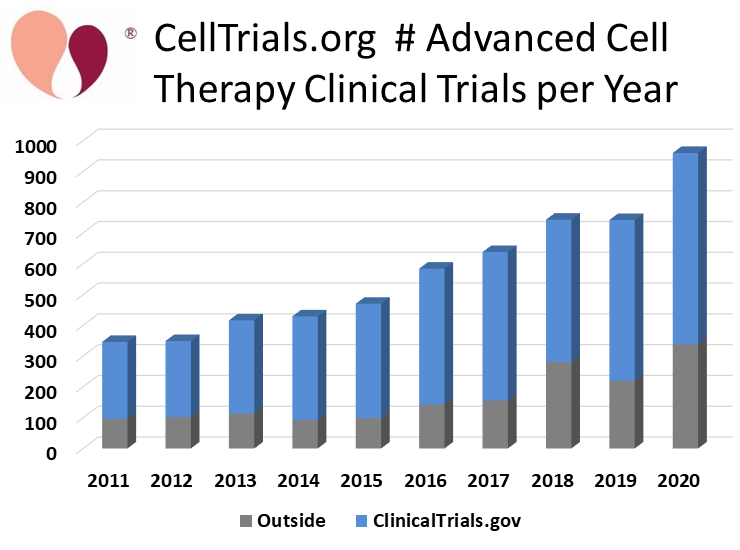
 CellTrials.org reports that 960 new clinical trials of advanced cell therapy were registered worldwide during 2020, a 29% increase over the number of trials during 2019 or 2018. Our report on the surging number of clinical trials is the first hard data on the pace of clinical research in the cell therapy field over the past year. Recently, multiple reports have tracked the pace of investment activity as well as mergers and acquisitions in the biotech field1-3.
CellTrials.org reports that 960 new clinical trials of advanced cell therapy were registered worldwide during 2020, a 29% increase over the number of trials during 2019 or 2018. Our report on the surging number of clinical trials is the first hard data on the pace of clinical research in the cell therapy field over the past year. Recently, multiple reports have tracked the pace of investment activity as well as mergers and acquisitions in the biotech field1-3.
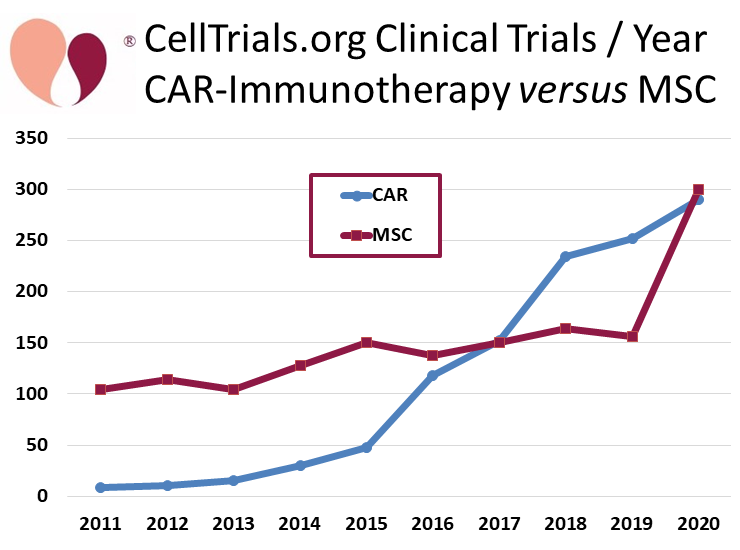
The big story in the cell therapy field that has been so far been overlooked is that the number of clinical trials with mesenchymal stromal cells (MSC) nearly doubled this past year, from 156 trials in 2019 to 300 trials in 2020. A graph versus time that compares the annual number of MSC trials versus CAR-immunotherapy trials demonstrates this dramatic jump.
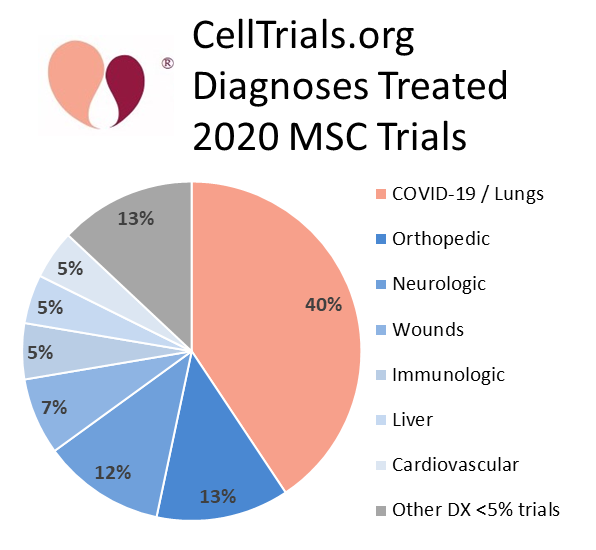
Our pie chart of the diagnostic indications treated by the 2020 MSC clinical trials shows that the largest category is pulmonary conditions. There were 116 MSC clinical trials registered in 2020 that treated COVID-19 and/or ARDS. This trend isunprecedented, like so many other things in 2020, because previous charts of the diagnoses treated by MSC clinical trials have always shown neurologic conditions to be the top category4-6. Hence, the trials that are specifically for or closely related to COVID-19 account for about 80% of the increased number of MSC clinical trials.
The impact of COVID-19 on clinical trials during 2020 was multi-faceted. It has been reported that COVID-19 caused a halt to thousands of trials impacting millions of patients7. At the same time, so many cell therapy developers rushed to repurpose their product pipeline to combat COVID-19, that there was a significant increase in new clinical trials of advanced cell therapy worldwide.
CellTrials.org compiles clinical trials from 16 national registries now; over time we are finding that additional countries have launched clinical trial registries. Whenever a trial is simultaneously posted to both the well-known US registry ClinicalTrials.gov and another national registry, we credit it to ClinicalTrials.gov. Among the new 2020 trials, 65% are registered on ClinicalTrials.gov, 21% on the Chinese registry ChiCTR, and the remaining 14% were listed elsewhere. We attribute 501 (52%) of the new 2020 trials to immunotherapy versus 459 (48%) to regenerative medicine.
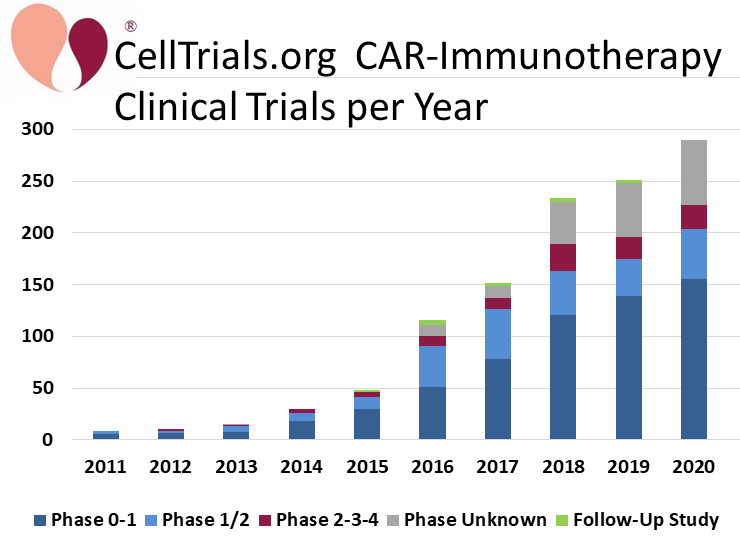
CellTrials.org data shows that 290 clinical trials of CAR-immunotherapy were registered in 2020, an increase of 16% over 2019. The CAR-immunotherapy field has also received a lot of media attention this past year, due to the FDA approval of another three autologous CAR-T therapies, making a total of five approved to date8. We display the number of trials per year over the past decade, color coded by trial phase.
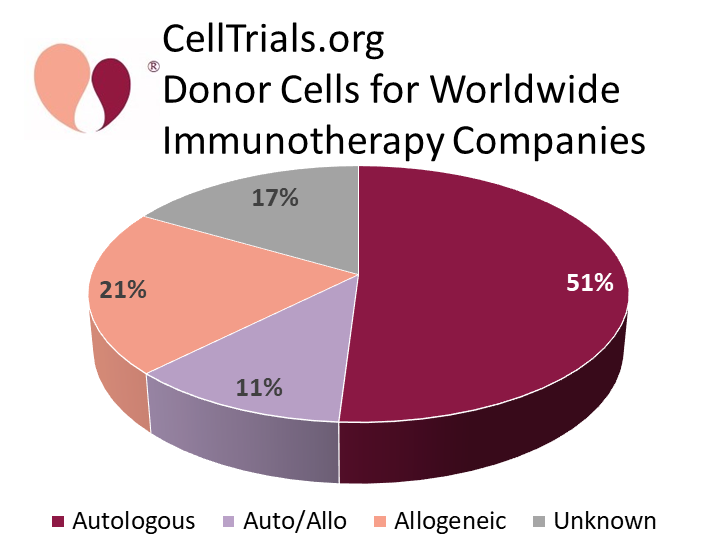
Over the year from Feb. 2020 to Feb. 2021, the number of worldwide companies developing cellular immunotherapy products increased from 357 to 451. However, the number of companies that are developing strictly autologous therapies remains roughly half of the total, from 54% a year ago to 51% now. It is important to note that immunotherapy research and development is not all focused on CAR-T products. For example, among the 143 companies outside China with strictly autologous products, 59% have no CAR-T products in their pipeline. The increasing number of companies and products in the immunotherapy field is not purely driven by CAR-T constructs, but reflects the potential applications of many types of immune cells.
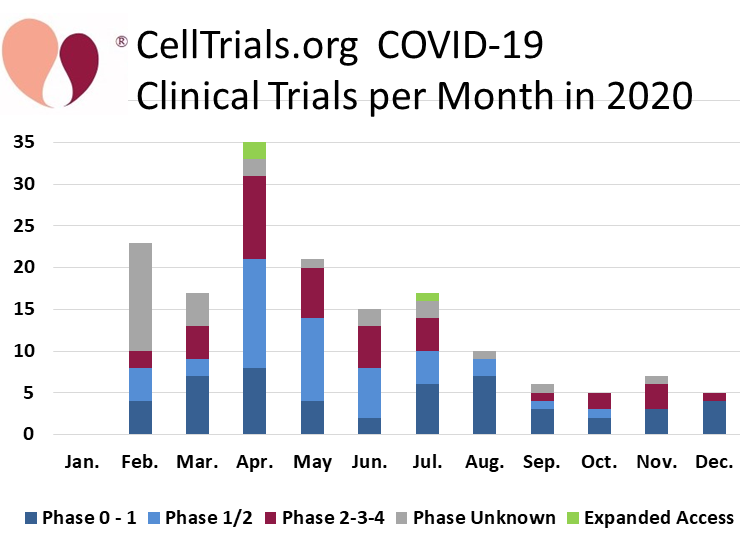 During the Coronavirus pandemic, CellTrials.org launched a community service of providing a free download of all cell therapy trials that are treating COVID-19. This includes trials of prophylactic treatments, interventions for patients with mild to severe COVID-19 infections, and therapies for COVID-19 survivors with long haul symptoms. Starting in February 2020 through December of that year, 161 cell therapy trials were registered worldwide to fight COVID-19. During the first half of 2020, we reported that 53% of the COVID-19 cell therapy trials were using cells derived from perinatal sources, but for the year as a whole the perinatal fraction dropped to 42%9.
During the Coronavirus pandemic, CellTrials.org launched a community service of providing a free download of all cell therapy trials that are treating COVID-19. This includes trials of prophylactic treatments, interventions for patients with mild to severe COVID-19 infections, and therapies for COVID-19 survivors with long haul symptoms. Starting in February 2020 through December of that year, 161 cell therapy trials were registered worldwide to fight COVID-19. During the first half of 2020, we reported that 53% of the COVID-19 cell therapy trials were using cells derived from perinatal sources, but for the year as a whole the perinatal fraction dropped to 42%9.
We display the number of COVID-19 cell therapy trials per month of 2020, color coded by trial phase. One might expect that the COVID-19 trials were predominantly early phase studies, but in fact 24% of the 161 trials are phase 2 to 3 (none were phase 4), and another 3 trials are expanded access programs. By comparison, 8% of the 290 CAR-immunotherapy trials in 2020 were phase 2 or higher. Hence the fraction of phase 2 or higher clinical trials was three times more for COVID-19 cell therapy than for CAR-immunotherapy trials in 2020. What does that reflect? We know that CAR-immunotherapy requires gene modification, whereas 116 (72%) of the cell therapies for COVID-19 relied purely on infusions with mesenchymal stromal cells (MSC) prepared from various sources with various isolation methods. We also know that 93% of the CAR-immunotherapy trials were based in either the United States or China, whereas only 50% of the COVID-19 trials were located in those two countries. The percentage of later phase COVID-19 trials may simply reflect the eagerness of regulatory authorities to approve clinical trials during a pandemic, especially given that MSC infusions have a well-established safety profile4-6.
The year 2020 broke many records in the field of cell therapy, both because of and despite the Coronavirus pandemic, and it remains to be seen which of these trends continue into 2021.
References
- Al Idrus A. In the face of COVID-19, cell and gene therapy space shows 'remarkable resilience': report. FierceBiotech Published 2020-08-06
- Cameron T, Morrison C. 2020 biotech IPOs shatter all the records. Nature BioBusiness Published 2021-01-20
- Langley K. Biotech Stocks Fall Out of Favor After Disappointing Trial Results, Big Rally. Wall Street Journal Published 2021-04-21
- Can A, Celikkan FT, Cinar O. Umbilical cord mesenchymal stromal cell transplantations: A systemic analysis of clinical trials. Cytotherapy 2017; 19(12):1351-1382.
- Couto PS, Shatirishvili G, Bersenev A, Verter F. First decade of clinical trials and published studies with mesenchymal stromal cells from umbilical cord tissue. Regen Med 2019; 14(4):309-319.
- Kabat M, Bobkov I, Kumar S, Grumet M. Trends in mesenchymal stem cell clinical trials 2004‐2018: Is efficacy optimal in a narrow dose range? Stem Cells Transl Med 2019; 9(1):17-27.
- Carlisle BG. Clinical Trials That Were Terminated, Suspended or Withdrawn Due to Covid-19. Open Science Framework Published 2020-05-07
- Verter F. Immune Cell Banking. Parent's Guide to Cord Blood Foundation News Published 2021-04
- Verter F, Couto PS. Development of COVID‐19 Therapies from Birthing Tissues and Cord Blood. Stem Cells Transl Med 2020; 9(S1):S15.